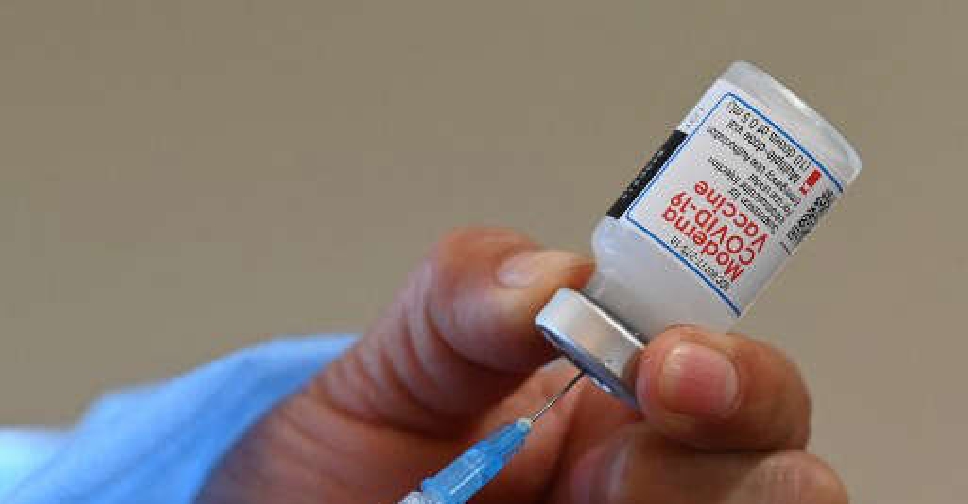
Britain's health regulator has approved Moderna's COVID-19 vaccine for use in children aged 12 to 17 years.
The approval comes more than two months after Pfizer and German partner BioNTech's COVID-19 vaccine got regulatory nod for use in children aged 12 to 15.
Moderna's vaccine was recommended for use in adolescents by European regulators in July and is awaiting US authorisation.
It is currently approved for people over the age of 18 in the UK.
Britain's Joint Committee on Vaccination and Immunisation (JCVI) gave the go-ahead on August 4 for 16 and 17-year-olds to get their first dose of Pfizer's COVID-19 vaccine ahead of schools returning in September.
JCVI will make a decision on whether the vaccine will be deployed or not.




 No evidence alleged Bondi gunmen received military training in Philippines
No evidence alleged Bondi gunmen received military training in Philippines
 At least 12 killed in Nigeria mining site attack
At least 12 killed in Nigeria mining site attack
 Russian attack on Ukraine's central Cherkasy injures six, causes blackouts
Russian attack on Ukraine's central Cherkasy injures six, causes blackouts
 UN, aid groups warn Gaza operations at risk from Israel impediments
UN, aid groups warn Gaza operations at risk from Israel impediments
 Israel approves natural gas deal with Egypt, Netanyahu says
Israel approves natural gas deal with Egypt, Netanyahu says




